These sensors use chemical reactions to detect and measure the presence of various substances, and are commonly used in gas detection and environmental monitoring.
Electrochemical sensors are devices used to detect and measure various chemical substances by converting a chemical reaction into an electrical signal. They are widely used in various industries, including environmental monitoring, healthcare, food and beverage, and industrial safety.
electrochemical sensors offer a reliable and sensitive means of detecting and measuring various chemical substances. Their ability to convert chemical reactions into electrical signals makes them invaluable tools in many industries where accurate and real-time chemical analysis is required.
Electrochemical sensors structure
Electrochemical sensors typically consist of three main components: a working electrode, a counter electrode, and a reference electrode. These electrodes are immersed in an electrolyte solution, which facilitates the chemical reactions(the reaction between an electrode and analyte).

The working electrode is typically made of a chemically inert material such as gold, platinum, or carbon. It is coated with a sensing material that selectively interacts with the target analyte.
The reference electrode provides a stable reference potential against which the working electrode’s potential can be measured. Common reference electrodes include silver/silver chloride (Ag/AgCl) or saturated calomel electrode (SCE).
The electrolyte is a conductive solution that allows ions to move between the working and reference electrodes, completing the electrochemical circuit.
Electrochemical sensors types and their working principle
The working principle of electrochemical sensors can vary depending on the type of sensor. Here is a general overview of how some common types work:
Amperometric sensors
These sensors measure the current generated by a redox reaction at the working electrode. They are widely used for the detection of gases, ions, and biomolecules. For example, amperometric gas sensors can detect gases like carbon monoxide (CO) or nitrogen dioxide (NO2). In amperometric sensors, a voltage is applied between the working and reference electrodes. When the target analyte comes in contact with the working electrode surface, a redox reaction occurs, resulting in a current flow proportional to the analyte concentration. The current is measured as an electrical signal, and its magnitude indicates the analyte concentration.

A redox reaction, also known as a reduction-oxidation reaction, is a chemical reaction in which there is a transfer of electrons between two species. One species undergoes oxidation (loses electrons) while the other undergoes reduction (gains electrons). The term “redox” is derived from the combination of these two processes: reduction and oxidation.
In a redox reaction, there are two components:
1. Oxidation: Oxidation occurs when a species loses electrons. This process results in an increase in the oxidation state or a loss of electrons from the species. The species that undergoes oxidation is called the reducing agent, as it facilitates the oxidation of another species.
2. Reduction: Reduction occurs when a species gains electrons. This process leads to a decrease in the oxidation state or a gain of electrons by the species. The species that undergoes reduction is called the oxidizing agent, as it facilitates the reduction of another species.
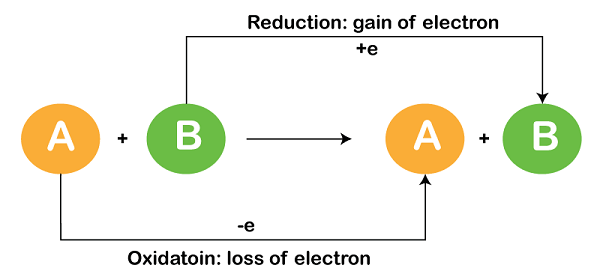
The transfer of electrons between the oxidizing and reducing agents is what characterizes a redox reaction. Electrons flow from the reducing agent to the oxidizing agent through an electrical circuit or, in the case of electrochemical reactions, through an electrode.
Redox reactions are fundamental in various biological, chemical, and electrochemical processes. They are responsible for energy production in cells, corrosion reactions, combustion processes, and many other essential reactions in nature and industry.
Potentiometric sensors
Potentiometric sensors are often used for pH measurement, as well as ion selective electrodes (ISEs) for detecting specific ions like sodium (Na+), potassium (K+), or chloride (Cl-).
Potentiometric sensors measure the potential difference between the working and reference electrodes. When the target analyte interacts with the sensing material on the working electrode, it causes a change in the potential difference. This change in potential is measured and correlated to the analyte concentration.
Conductometric Sensors
Conductometric sensors measure changes in electrical conductivity caused by the interaction between the analyte and the working electrode. The sensing material on the working electrode undergoes a chemical reaction with the analyte, leading to a change in conductivity.
They are commonly used for gas sensing applications, such as detecting volatile organic compounds (VOCs) or ammonia (NH3).
Biosensors
Biosensors are a type of electrochemical sensor that utilizes biological components, such as enzymes or antibodies, to selectively detect and measure specific biomolecules. When the target biomolecule binds to the sensing material, it triggers a biochemical reaction that generates an electrical signal. The magnitude of the signal is proportional to the concentration of the target biomolecule.
They are used in various applications, including glucose monitoring, DNA sequencing, and medical diagnostics.

These are just a few examples of the many types of electrochemical sensors available. Each sensor type has its own advantages and limitations, making them suitable for different applications and analytical requirements.
Electrochemical sensors require calibration to establish a relationship between the measured current and the concentration of the analyte. This is typically done by exposing the sensor to known concentrations of the analyte and creating a calibration curve that relates the sensor’s output signal to the analyte concentration.
Electrochemical sensors have a wide range of applications. They are commonly used for gas detection (e.g., carbon monoxide, oxygen), environmental monitoring (e.g., water quality analysis), medical diagnostics (e.g., glucose monitoring), and industrial process control.