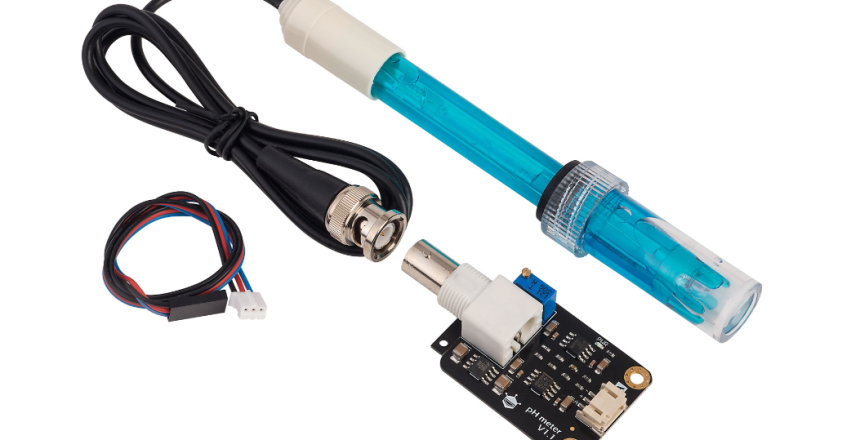
A pH sensor measures the acidity or alkalinity of a solution. It is used in various fields such as chemistry, environmental science, and agriculture. Types include glass electrode pH sensors, ISFET pH sensors, and test strip pH sensors. Applications include water quality testing, food and beverage production, swimming pool maintenance, and scientific research.
A pH sensor, is a device used to measure the acidity or alkalinity of a solution.
pH is a measure of the concentration of hydrogen ions (H+) in a solution, and it is expressed on a logarithmic scale ranging from 0 to 14. A pH value of 7 is considered neutral, below 7 is acidic, and above 7 is alkaline.

The basic structure of PH sensors and how they work
pH sensors typically consist of three main components: a sensing element, a reference element, and a junction. Let’s take a closer look at each component:
Sensing Element
The sensing element is the part of the pH sensor that comes into contact with the solution being measured. It is responsible for detecting changes in hydrogen ion concentration and converting them into an electrical signal. The most common type of sensing element is a glass membrane electrode. It is made of a thin layer of special glass that is sensitive to pH changes. When exposed to a solution, the glass membrane generates a tiny voltage that is proportional to the pH level.
Reference Element
The reference element is used to provide a stable reference potential against which the pH measurement is made. It ensures that the pH sensor operates accurately and consistently. The reference element usually consists of either a saturated calomel electrode (SCE) or a silver/silver chloride electrode, both of which have a known stable potential. The reference electrode is filled with a reference electrolyte solution that maintains a constant potential regardless of changes in the sample solution.
Junction
The junction is a small opening or pathway that connects the reference element to the sensing element. It allows the reference electrolyte solution to come into contact with the sample solution, enabling the transfer of ionic potentials. The junction is designed to minimize clogging and contamination while allowing for ion exchange between the reference and sample solutions. Common types of junctions include ceramic, polymer, or porous frit.

These three components work together to measure the pH of a solution. When the sensing element comes into contact with a sample, it generates a voltage proportional to the difference in hydrogen ion concentration between the sample and the reference element. This voltage is then measured and converted into a pH reading by the pH meter connected to the sensor.
PH sensors different types
There are several types of pH sensors available, each with its own advantages and applications. Here are some common types:
Glass Electrode
A glass electrode is a type of electrochemical sensor that is commonly used in various applications, including pH measurement. It consists of a thin glass membrane and an internal reference electrode.
The glass membrane is the crucial part of the electrode. It is made of a specially formulated glass that is selectively permeable to certain ions, such as hydrogen ions (H+). This allows it to respond to changes in ion concentration, particularly in the case of pH measurement.
Inside the glass electrode, there is an internal reference electrode. It serves as a stable reference point against which the potential difference is measured. The reference electrode is typically filled with a solution containing a known concentration of reference ions, often silver/silver chloride (Ag/AgCl) or saturated calomel electrode (SCE).
The glass membrane of a glass electrode is specifically designed to be sensitive to changes in the ion concentration being measured.

For pH measurement, the glass electrode is sensitive to hydrogen ions (H+) in the solution. As the H+ concentration changes, it generates a corresponding potential difference across the glass membrane.
One of the primary applications of a glass electrode is pH measurement. By immersing the glass electrode in a solution, it reacts to the concentration of hydrogen ions present in the solution. This reaction generates a voltage, which is then converted into a pH value using a pH meter.
In addition to pH measurement, glass electrodes can also be used for other types of ion measurements, such as measuring the concentration of specific ions in solutions. These include measurements of fluoride, sodium, calcium, potassium, and more. Each application may require a different type of glass composition or specific calibration procedures.
glass electrodes require proper handling and maintenance to ensure accurate and reliable measurements. They should be calibrated regularly according to the manufacturer’s instructions and handled with care to avoid damage to the delicate glass membrane.
Ion-Selective Field Effect Transistor (ISFET)
An Ion-Selective Field Effect Transistor (ISFET) is a type of sensor used to measure ion concentrations in solutions. It combines the principles of a field-effect transistor (FET) with an ion-selective membrane.
An ISFET consists of a silicon-based FET structure with a gate electrode covered by an ion-selective membrane. The ion-selective membrane is typically made of a material that selectively interacts with specific ions, allowing for their detection.
The ion-selective membrane of an ISFET is designed to be sensitive to specific ions, such as hydrogen ions (H+) for pH measurement or other ions like sodium (Na+), potassium (K+), chloride (Cl-), and more. The presence of these ions causes a change in the electrical properties of the membrane, which affects the FET’s conductance or threshold voltage.
Unlike glass electrodes, which require a reference electrode and pH meter, ISFETs can directly measure ion concentrations without the need for an additional reference electrode. This makes them advantageous in terms of simplicity and miniaturization.
The most common application of ISFETs is pH measurement. By using an ion-selective membrane sensitive to hydrogen ions, ISFETs can accurately determine the pH of a solution.

ISFETs have a wide range of applications beyond pH measurement. They can be used for monitoring ion concentrations in various fields, including environmental monitoring, food and beverage industry, biomedical research, and chemical analysis.
ISFETs require careful handling and calibration to ensure accurate and reliable measurements. The ion-selective membrane can be susceptible to fouling or contamination, which may affect its performance.
Polymer-Based Sensors
Polymer-based pH sensors are a type of pH sensor that utilizes polymers as the sensing material. These sensors offer several advantages and have applications in various fields.
Polymer-based pH sensors work based on the principle of ion exchange between the target solution and the polymer sensing material. The polymer is designed to selectively interact with hydrogen ions (H+) present in the solution, leading to changes in its electrical properties.
Polymer-based pH sensors can cover a wide pH range, from highly acidic to highly alkaline solutions. Depending on the specific polymer used, these sensors usually have good sensitivity and accuracy across a broad pH spectrum.
Polymers offer flexibility in terms of sensor design, making them suitable for various applications. These sensors typically exhibit a fast response time, allowing for quick and real-time pH measurements. This makes them suitable for dynamic environments or applications requiring rapid pH monitoring.
Many polymer materials used in pH sensors are biocompatible, making them ideal for applications in the biomedical and healthcare sectors. They can be used for monitoring pH levels in biological fluids, such as blood, saliva, or even inside living organisms.
Differential Electrode
Differential electrode pH sensors consist of two electrodes with different characteristics. One electrode measures the pH, while the other acts as a reference electrode. This design helps eliminate errors caused by temperature variations, electrical interference, or contamination.
Combination pH Electrode
Combination electrodes include both the pH-sensitive glass membrane electrode and a reference electrode integrated into a single unit. These sensors are convenient, easy to use, and suitable for general-purpose pH measurements.
It’s important to select the right pH sensor type based on the specific requirements of your application, such as the nature of the sample, temperature range, chemical compatibility, accuracy needs, and environmental conditions.